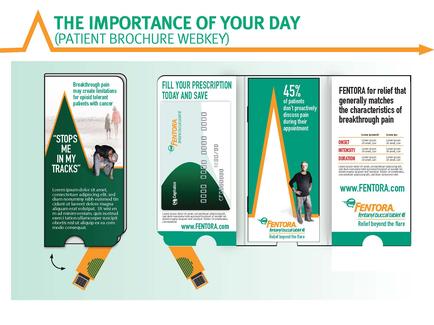
A slide from a presentation about the Butrans (buprenorphine) Transdermal System. The slide has a purple background with white text. On the left side of the slide there is a title that reads "Butrans is indicated for the management of moderate to severe chronic pain in patients requiring a continuous around-the-clock opioid analgesic for an extended period of time". Below the title there are three images of Butrans tablets. The tablets are white with blue and yellow accents.
The first image on the slide explains that Butrans is a Schedule III a single-entity opioid analgesics and that it does not contain APAP an NSAID or a COX inhibitor. The second image shows the tablets with the text "Butrans is a transdermal system providing systemic delivery of buprenorphine continuously for 7 days". The third image shows a description of the product which states that it is not available in the United States.

Category
Source 1 of 2
-
Date
2011
Collection
-
Date
2011
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.