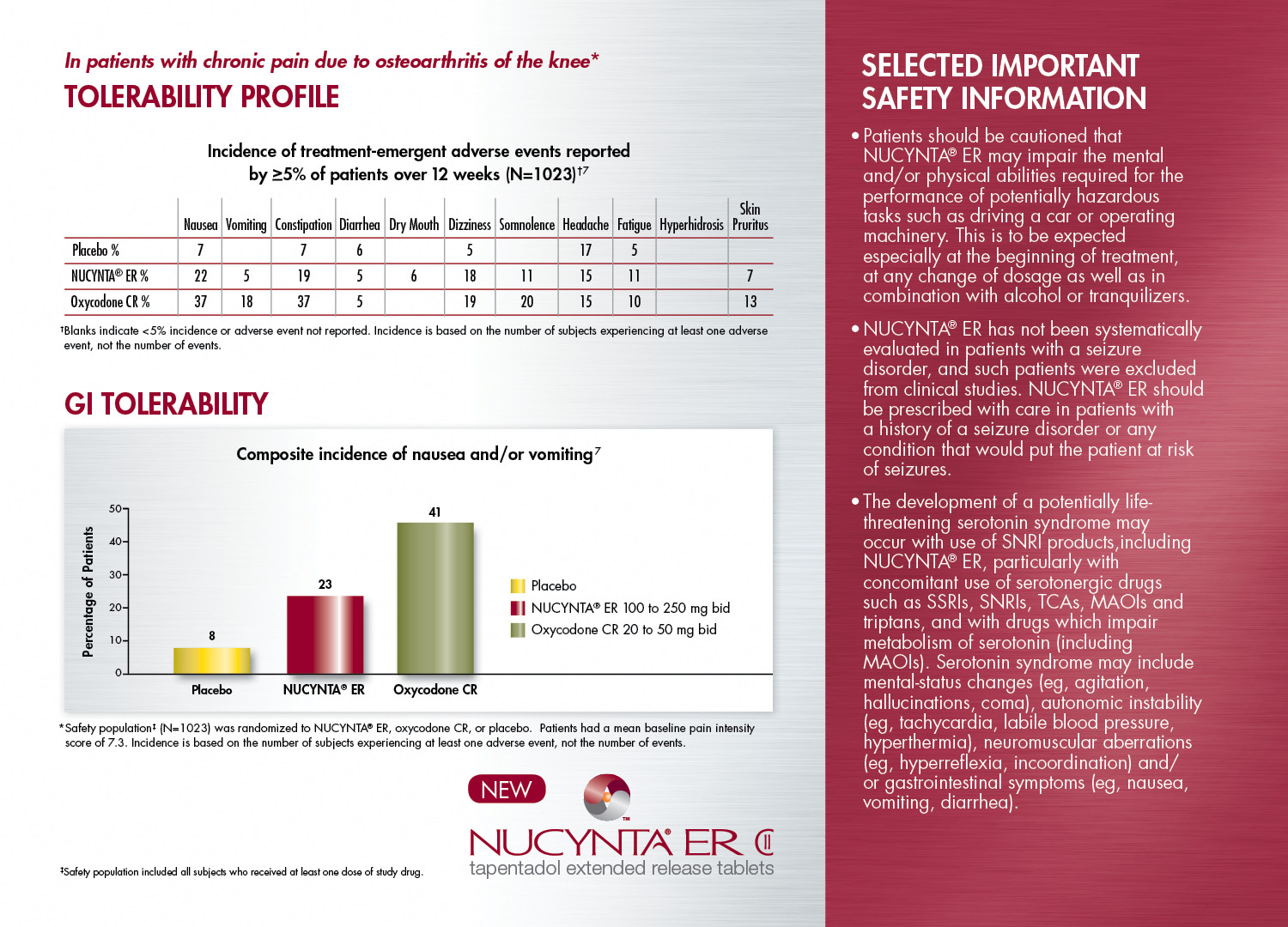
Information about NUCYNTA ER. It is titled "In patients with chronic pain due to osteoarthritis of the knee". There is a table and a bar graph below the title.
The table is titled "Tolerability Profile" and has the subtitle "Incidence of treatment-emergent adverse events reported by greater than or equal to 5% of patients over 12 weeks (N=1023)". The table shows various adverse events and the percentage of incidences reported per event for the placebo NUCYNTA ER and Oxycodone CR.
The bar graph is titled "GI Tolerability" and has the subtitle "Composite incidence of nausea and/or vomiting". The x-axis has sections for placebo NUCYNTA ER and Oxycodone CR. The y-axis is labeled "Percentage of Patients" with a range of values between 0 and 50. The bars on the chart show that 8% of patience had incidences on placebo 23% on NUCYNTA ER and 41% on Oxycodone CR. The NUCYNTA ER logo is visible below the bar graph.
The image also shows a red side bar with selected important safety information.
Description
Category
-
Date
2007
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.