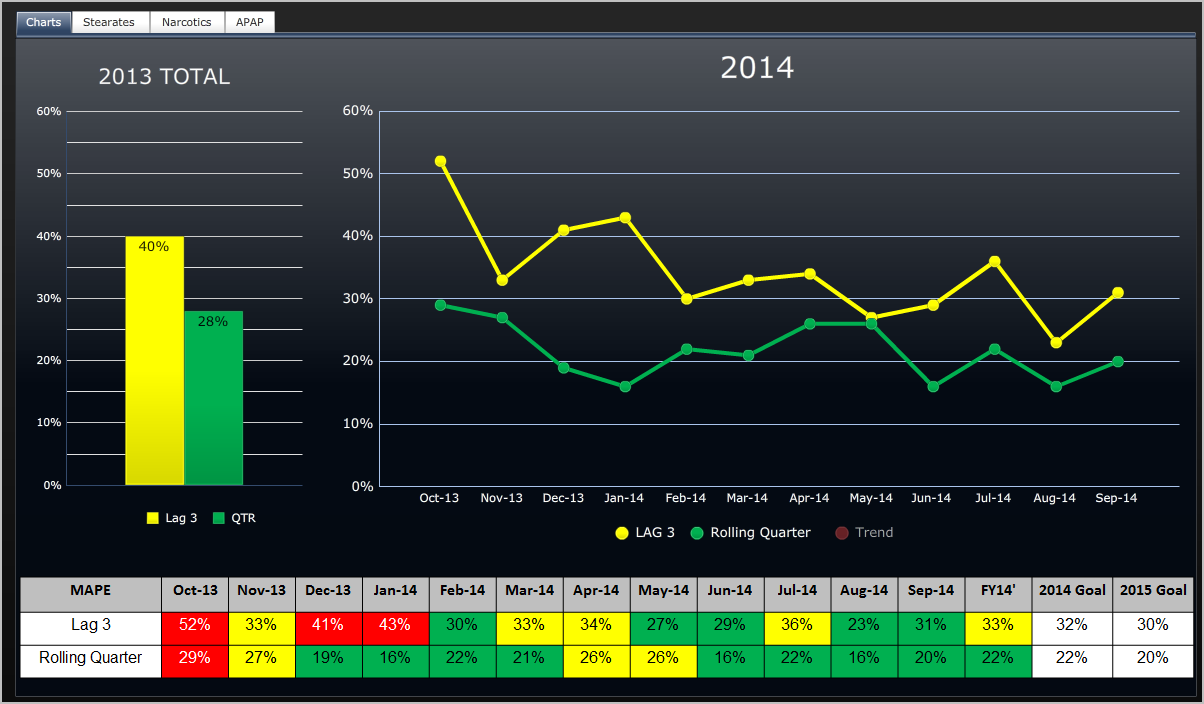
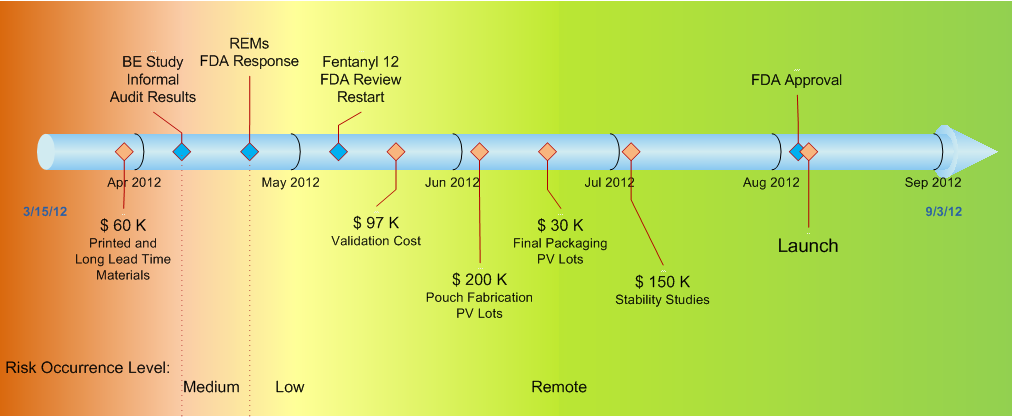
A timeline of the FDA approval process and manufacturing of for a product possibly fentanyl. The timeline goes from 3/15/12 to 9/3/12. It includes items (in no particular order) such as: BE Study FDA Response; BE Study FDA Audit Results; Fentanyl 12 FDA Review Restart; FDA Approval; $60k printed and Long Lead Time Materials; Pouch Fabrication PV Lots; Stability Studies; and Launch. The timeline is color coded to indicate when a risk occurrence level is medium low or remote.
Description
Type
Category
-
Date
2009
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.