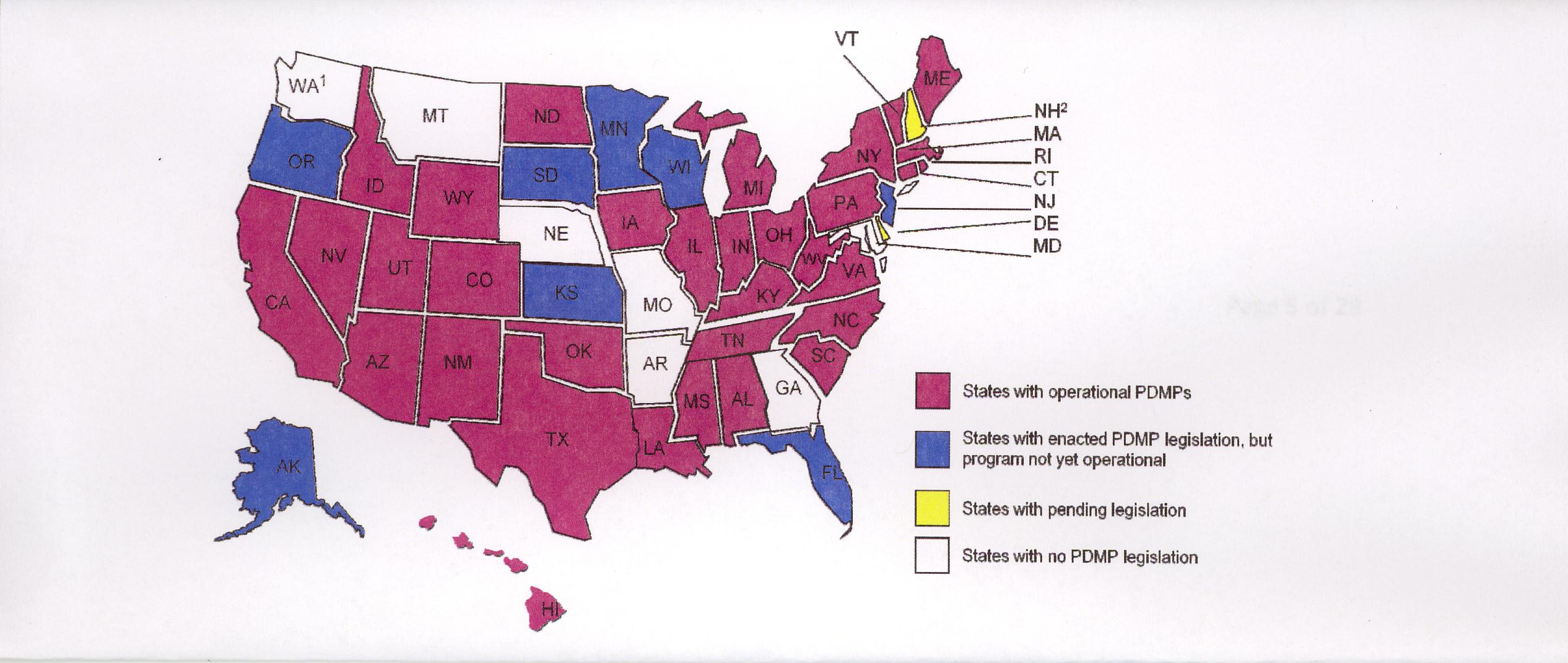
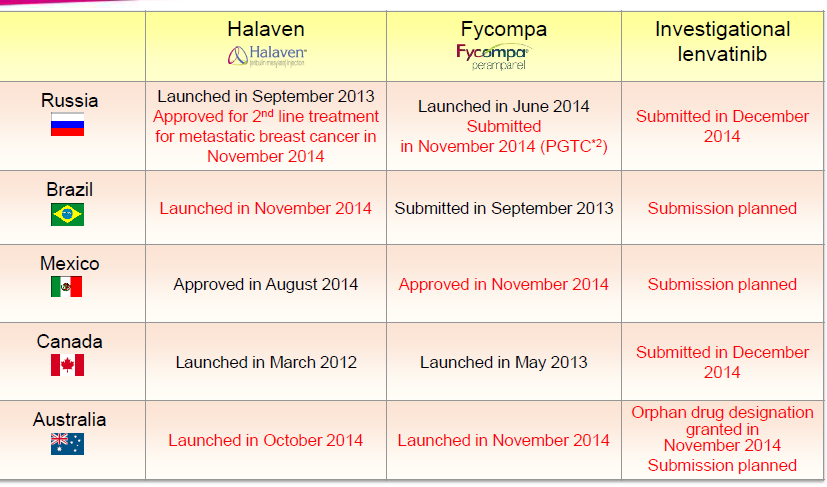
A table that shows the results for product submissions approvals and launches for Halaven Fycompa and Investigative Lenvatinib in Russia Brazil Mexico Canada and Australia.
Description
Category
Source 1 of 3
-
Date
2015
Collection
-
Date
2015
Collection
-
Date
2015
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.