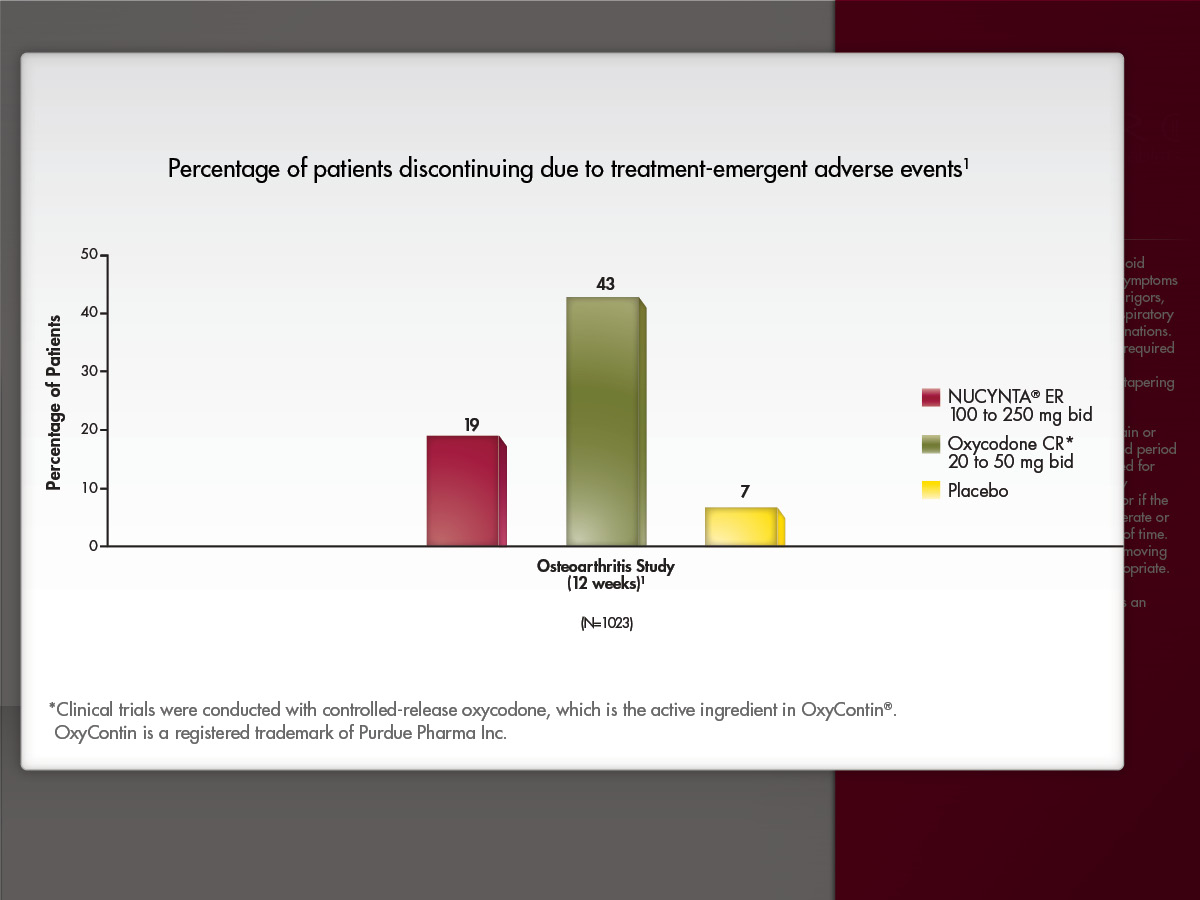
A bar graph titled "Percentage of patients discontinuing due to treatment-emergent adverse events". The x-axis of the graph is labeled "Osteoarthritis Study (12 weeks) N = 1023". The y-axis is labeled "Percentage of patients" with a range of values from 0 to 50. The bars on the graph represent NUCYNTA ER 100 to 250 mg bid (19%) Oxycodone CR 20 to 50 mg bid (43%) and placebo (7%). Text at the bottom of the image reads: "Clinical trials conducted with controlled-release oxycodone which is the active ingredient in OxyContin®. OxyContin® is a registered trademark of Purdue Pharma Inc."
Type
Category
-
Date
2007
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.










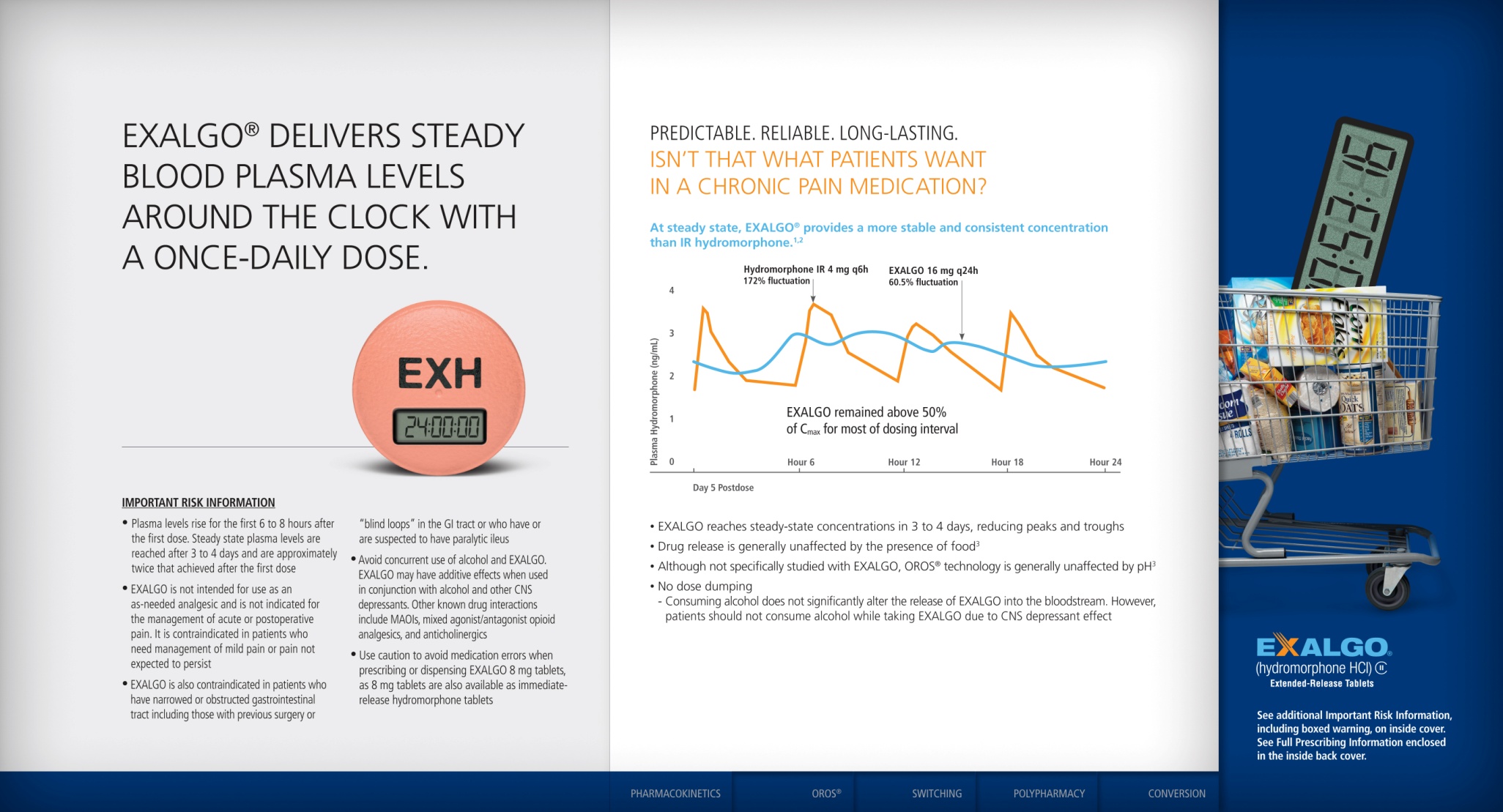
![A flow chart describing the pain management process from a physician's perspective in 5 sections accompanied by 7 annotations (marked by stars pointing to text boxes). The sections are Origin Evaluation and Diagnosis Treatment Choices [something to do with insurance coverage for prescriptions?] and Persistency. The annotations appear to refer to ways that Mallinckrodt Pharmaceuticals could increase the awareness sales and profitability of Exalgo. The background is white with graphic elements in blue gray green and yellow with text in black red and green.](https://oida-resources-images.azureedge.net/public/full/c3a89e52-3266-47c0-9ed9-fe67133608e2.png)