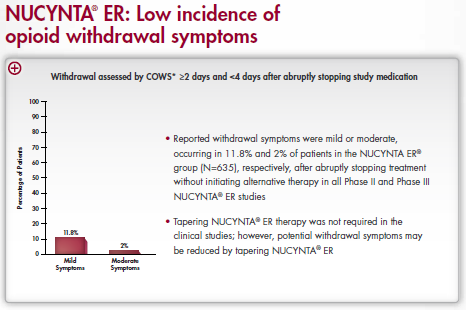
Information about NUCYNTA ER. It is titled "NUCYNTA ER: Low incidence of opioid withdrawal symptoms". There is a bar graph at the center of the image titled "Withdrawal assessed by COWS greater than or equal to 2 days and less than 4 days after abruptly stopping study medication". The x-axis has sections for mild and moderate symptoms. The y-axis is labeled "Percentage of Patients" with a range of values between 0 and 100. The bars on the chart show that 11.8% of patients had mild symptoms and 2% had moderate symptoms.
Text to the right of the graph indicates that reported withdrawal symptoms were mild or moderate occurring in 11.8% and 2% of the patients in the NUCYCYNA ER group (N=635) respectively after abruptly stopped treatment without initiating alternative therapy in all Phase II and Phase III NUCYNTA ER studies. It also indicates that tapering NUCYNTA ER therapy was not required by the clinical studies; however potential withdrawal symptoms may be reduced by tapering NUCYNTA ER.
Description
Category
-
Date
2010
Collection
We encourage you to view the image in the context of its source document(s) and cite the source(s) when using these images. However, to cite just this image alone, click the “Cite This Image” button and then paste the copied text.